Introduction
Animal health and welfare are essential for efficient lamb production and disease control is a vital component of a successful sheep farming business. Treatment of disease is not as effective or as economical as prevention.

Fig 1:Vaccination against clostridial diseases is an essential component of the flock health plan.

Fig 2: Timely vaccination of store lambs against clostridial diseases at weaning.
Poor health status and subclinical disease can be a major cause of financial losses on many sheep farms. The impact of many diseases can be avoided or minimised by reducing exposure to disease pathogens (effective biosecurity) and implementing effective vaccination and parasite control programmes. These programmes will be designed for each individual sheep farm and included in the flock health plan by the farmer's veterinary practitioner.
When treatments are necessary there is a joint responsibility between the veterinary surgeon and the farmer to ensure that all drugs, but especially all antimicrobial drugs, are used correctly and responsibly such that all legal requirements are met.

Fig 3: Farmers must consult their veterinary practitioner regarding the correct choice of antimicrobial drug, frequency of administration, and duration of therapy for joint ill in lambs.
Prescribing categories
Under the 2005 legislation drugs administered to sheep were re-classified replacing POM, PML, GSL categories with:
- Prescription Only Medicine-Veterinarian (POM-V)
- Prescription Only Medicine-Veterinarian, Pharmacist, Suitably Qualified Person (POM-VPS)
- Non-Food Animal-Veterinarian, Pharmacist, Suitably Qualified Person (NFA-VPS)
- Authorised Veterinary Medicine-General Sales List (AVM-GSL)
POM-V
POM-Vs can only be prescribed by veterinary surgeons for administration to animals under their care of a veterinary surgeon. The Royal College of Veterinary Surgeons requires that a number of criteria should be met for an animal to fall into this category:
-
The veterinary surgeon should have been given responsibility for the animal's health by its owner or guardian.
-
The veterinary surgeon should have performed a clinical examination of the animal for the purpose of diagnosis or prescription, or have visited the premises in which the animal is kept sufficiently often and recently enough to have sufficient personal knowledge to make a diagnosis and prescribe for the animal in question.

Fig 4: The veterinary surgeon should have visited the premises in which the animal is kept sufficiently often and recently enough to have sufficient personal knowledge to make a diagnosis and prescribe for the animal in question.
POM-Vs may also be supplied by a pharmacist or another registered veterinary surgeon but only on production of a written prescription from the animal owner's veterinary surgeon.

Fig 5: No response to drugs administered by the farmer to this weaned Scottish Blackface ram lamb.

Fig 6: The same weaned Scottish Blackface ram lamb (see above) three days after veterinary treatment. Examination by a veterinary practitioner is essential in many cases.
POM-VPS
A clinical assessment is not necessarily required prior to prescription of POM-VPS. This category corresponds closely to the former PML (Pharmacy Merchants List) group of medicines. Medicines in this category can only be prescribed by a registered qualified person (RQP). A RQP is defined as a:
- Registered Veterinary Surgeon
- Registered Pharmacist
- Registered Suitably Qualified Person (SQP) for that species e.g. for the treatment of farm animals only (QL-SQP).

Fig 7: These ewes had been drenched on several occasions with an anthelmintic (wormer) - they were suffering from liver fluke!
AVM-GSL
Medicinal products under this category may be supplied by any retailers (including supermarkets and pet shops) with no restrictions on supply. This category corresponds closely to the former GSL (General Sales List).
Health and Safety requirements for medicines
In addition to the considerations above, medicines are also controlled by the Control of Substances Hazardous to Health (COSSH) Regulations 2002 (enacted under the Health and Safety at Work Act 1974). COSHH regulations relate to work involving substances that are deemed to be hazardous to health and includes veterinary medicines and other substances produced by animals (blood, tissue, urine, faeces). It is the employer's responsibility to perform a risk assessment of each of these substances. Manufacturers of veterinary products provide a product safety data sheet to aid such risk assessment. The employer must prevent or control exposure of employees to these substances by information, instruction and training.
Withdrawal periods for drugs (Food producing animals)
The Animals, Meat and Meat Products (Examination for Residues and Maximum Residue Limits) Regulations 1997 control residues of animal medicines in food producing animals. Maximum Residue Limits (MRL) are set which aim to avoid toxicity in man and technical problems for the food producing industries. Under EU legislation the MRL is defined as:
Maximum concentration of residue resulting from administration of an animal medicine which is legally permitted in the Community or recognised as acceptable in or on a food.
Withdrawal periods for meat are listed on the data sheet accompanying the drug and must be strictly observed. Withdrawal periods are defined as:
The time between the last dose given to the animal and the time when the level of residues in the tissues (muscle, liver, kidney, skin/fat) or products (milk, eggs, honey) is lower than or equal to the MRL.
Withdrawal periods are given for time after administration to slaughter (meat production). Where a withdrawal period is not given for a species a minimum of the following "standard" withdrawal periods should be adopted; 28 days for meat. Additionally, some organic food schemes require the doubling or tripling of data sheet and standard withdrawal periods.
Storage of veterinary medicines
Most of the requirements for the storage of veterinary medicines are based on common sense. The important points are:
- Store in accordance with the manufacturer's instructions.
- Refrigeration must be available and maintained between 2°C and 8°C. Refrigerators should be fitted with a maximum / minimum thermometer to allow monitoring of the temperature.
- The designated storage area should not be accessible to the public.
- Storage areas should be kept clean and should be well ventilated. Eating or drinking should be forbidden in this area.
- Dates of delivery should be logged and marked on products and for multi-use products date of first use should be marked on the product.
Practical points for handling medicines
- Direct contact between the skin of the person dispensing the drug and the drug itself should be avoided. This can be achieved through wearing protective clothing, such as disposable gloves.
- The data sheet should always be consulted.
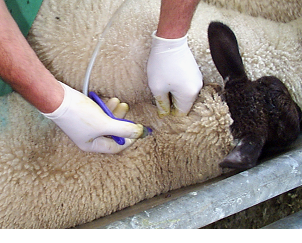
Fig 8: Operator wearing protective disposable gloves during routine vaccination.
Sheep products that can pose certain risks to operators during handling and administration include orf vaccine , Chlamydophila abortus vaccines, Toxoplasma gondii vaccine, and footrot vaccine.
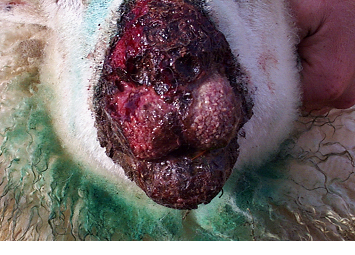
Fig 9: Vaccines used to prevent orf must be handled with care.
Parenteral administration of medicines.
Medicines can be given by intravenous, intramuscular and subcutaneous injection. A new sterile needle and syringe should be used on every occasion.
The only exception to single syringe/needle use is the administration of a vaccine to a number of sheep using an automatic syringe. In this situation it is essential that the fleece is clean, free from contamination, and dry. Vaccination of dirty or wet sheep should be delayed until the fleece is clean and dry otherwise there is the risk of introduction of bacteria with the vaccine and the development of an abscess at the injection site. A maximum of 20-25 sheep should be injected with one needle before it is changed (this number will often correspond to the number of doses in the bottle).
The sheep must be adequately restrained for the injection procedure, usually in a race or small pen.
The correct size of hypodermic needle should be used
Lambs less than 10 kg 21 gauge 5/8 inch
Lambs 10 to 20 kg 20 gauge 1 inch
Lambs 20 to 40 kg 19 gauge 1 inch
Adult sheep 18 gauge 1 inch
The volume injected at a single site must not exceed that stated in the data sheet. Only administered by the stated route(s). An accurate liveweight measurement must be used either by weight crate or weigh band. Underestimates of bodyweight may lead to under-dosing and the medicine not being wholly effective. This situation is most likely when treating a group of animals with a wide range of weights where the average bodyweight is selected (e.g. drenching growing lambs with an anthelmintic drug).
When using medicines which are suspensions, such as penicillin preparations, thorough mixing is essential before administration.
When using the same bottle of medicine multiple times, a needle should be inserted through the rubber stopper and left in situ with the syringe attached to this needle to withdraw the drug. A new needle is attached to the syringe each time before injecting the drug into the sheep.
Subcutaneous and intramuscular injections
Subcutaneous injections are administered in areas where the skin is loose (mainly on the side of the neck or behind the shoulder).
Grasp a fold of skin and slide the needle through the skin parallel to the animal's neck or trunk. This method will avoid penetration of underlying muscle or injection into the chest cavity. The needle should be inserted several inches from the operator's hand to avoid accidental self-injection. The plunger of the syringe should always be pulled back to ensure that the needle point is not located within a blood vessel. Ensure that the needle point does not emerge from the skin fold. Slight resistance when depressing the syringe plunger will indicate that the solution is being injected subcutaneously and not through the skin fold and onto the skin surface.
The main site for intramuscular injection is the muscle mass of the neck. Alternatively, the muscles of the hind leg can be used. Draw up the solution for injection into the syringe. Insert the needle connected to the syringe into the muscle to the hub with a sharp action. Draw back to check for absence of blood, then slowly inject the contents of the syringe over 10 seconds. Do not inject too quickly as this main cause pain to the animal.
Intravenous injection
This is the fastest route for drug administration, bypassing absorption and is commonly used by veterinary practitioners. However, rapid injection of a drug bolus may cause adverse reactions, such as collapse. Drugs administered intravenously include some antimicrobials, and mineral solutions such as calcium borogluconate. The jugular vein in the neck is the common site. Extravascular injection can lead to severe local tissue reaction overlying the site of injection. The use of dirty injection equipment introduces infection directly into the blood stream and may have fatal consequences.
Drenching
Small volumes of liquid (less than 30 mls) are frequently administered by mouth using a drenching gun (e.g. anthelmintics and flukicides, and some trace element drenches). The animal is suitably restrained with the hind quarters lodged so the sheep cannot move backwards away from the operator. The sheep's head is held with the chin up and the liquid slowly squirted over the back of the tongue. The sheep's head is released once it has swallowed the liquid.
Larger volumes can be administered by stomach tube (orogastric tube), most commonly to lambs.
Lambs
The stomach tube is passed slowly through the lamb's mouth and advanced when the lamb swallows. The diameter of the stomach tube is greater than the wind pipe so accidental fluid administration into the lungs is prevented.
The Veterinary Medicines Regulations 2013
Part 3
Records
Food-producing animals: proof of purchase of veterinary medicinal products
The keeper of a food-producing animal must keep proof of purchase of all veterinary medicinal products acquired for the animal (or, if they were not bought, documentary evidence of how they were acquired).
It is an offence to fail to comply with this regulation.
Food-producing animals: records of administration by a veterinary surgeon
A veterinary surgeon who administers a veterinary medicinal product to a food producing animal must either enter the following information personally in the keeper's records or give it to the keeper in writing (in which case the keeper must enter the following into those records) -
(a) the name of the veterinary surgeon;
(b) the name of the product and the batch number;
(c) the date of administration of the product;
(d) the amount of product administered;
(e) the identification of the animals treated;
(f) the withdrawal period.
It is an offence to fail to comply with this regulation.
Food- producing animals: records of acquisition and administration
When a veterinary medicinal product is bought or otherwise acquired for a food producing animal the keeper must, at the time, record-
(a) the name of the product and the batch number;
(b) the date of acquisition;
(c) the quantity acquired; and
(d) the name and address of the supplier.
At the time of administration (unless the administration is by a veterinary surgeon in which case the record must be in accordance with regulation 18) the keeper must record-
(a) the name of the product;
(b) the date of administration;
(c) the quantity administered;
(d) the withdrawal period; and
(e) the identification of the animals treated.
A keeper who disposes of any or all of the veterinary medicinal product other than by treating an animal must record-
(a) the date of disposal;
(b) the quantity of product involved; and
(c) how and where it was disposed of.
It is an offence to fail to comply with this regulation.
Food-producing animals: retention of records
The keeper of a food-producing animal must keep the documentation on the acquisition of a veterinary medicinal product and the records relating to the product for at least five years following the administration or other disposal of the product, irrespective of whether or not the animal concerned is no longer in that keeper's possession or has been slaughtered or has died during that period.
It is an offence to fail to comply with this regulation.
Veterinary healthcare waste
Controlled mainly by: Control Hazardous Waste (England and Wales) Regulations (HWR) 2005
| Items | Method of disposal | |
|
Sharps |
Needles, blades, broken contaminated glass |
"Sharps" bin, usually yellow with a white or red lid |
|
Pharmaceutical waste |
Used and empty medicines containers and used syringes Part used or expired drugs, empty bottles, vials and other pharmaceutical waste |
"Pharmy" bin usually a green or yellow container "DOOP" containers (Destruction Of Old Pharmaceuticals), usually green with a red lid. Return to veterinary surgery for disposal. |


